What is a systematic review?
"A systematic review attempts to collate all empirical evidence that fits pre-specified eligibility criteria to answer a specific research question. It uses explicit, systematic methods that are selected to minimize bias, thus providing reliable findings from which conclusions can be drawn and decisions made... Meta-analysis is the use of statistical methods to summarize and combine the results of independent studies." - Cochrane Collaboration
-
Cochrane HandbookThe official guide for Cochrane Reviews, the Handbook includes guidance on the standard methods applicable to every review (planning a review, searching and selecting studies, data collection, risk of bias assessment, statistical analysis, GRADE and interpreting results), as well as more specialized topics (non-randomized studies, adverse effects, complex interventions, equity, economics, patient-reported outcomes, individual patient data, prospective meta-analysis, and qualitative research).
-
IOM Standards for Systematic ReviewsThe Institute of Medicine (IOM) recommends 21 standards for developing high-quality systematic reviews of comparative effectiveness research. The standards address the entire systematic review process from the initial steps of formulating the topic and building the review team to producing a detailed final report that synthesizes what the evidence shows and where knowledge gaps remain.
-
JBI Manual for Evidence SynthesisFrom the Joanna Briggs Institute, this online manual has separate chapters devoted to the synthesis of different types of evidence and to address different types of review questions.
-
PRISMAPreferred Reporting Items for Systematic Reviews and Meta-Analyses. Includes checklists, flow diagrams, and other resources to help authors improve the reporting of systematic reviews and meta-analyses.
-
Systematic Review ToolboxA searchable database of tools to help with protocol development, screening, quality assessment, reporting standards, and guidelines.
-
Meeting the Review Family: Exploring Review Types and Associated Information Retrieval Requirements (A Sutton et al., 2019)Wondering what type of review is most appropriate for your topic/question? Check out this article that discusses the differences between reviews such as systematic, scoping, narrative, rapid, etc.
-
Systematic Review or Scoping Review? (Z Munn et al., 2018)Published in BMC Medical Research Methodology, this article provides guidance for authors when choosing between a systematic or scoping review approach.
-
Comparing Review Types (SCSU Library)Key details and differences between integrative, scoping, and systematic reviews.
-
Other Review Types (University of South Australia)Compares systematic, scoping, and narrative reviews.
-
Types of Reviews (Edith Cowan University)Compares different review types.
-
Difference Between Narrative and Systematic Reviews (DistillerSR)Describes differences in objectives and methodology.
-
Systematic and Scoping Reviews: A Comparison and OverviewArticle by Smith & Duncan (Semin Vasc Surg, 2022).
Getting started
I. Before carrying out your systematic review, search the literature (in PubMed, Scopus, Google Scholar, etc.) for any systematic reviews that have already been published on your topic or related to your topic. The benefits are two-fold:
(1) helps to ensure that the work has not already been done (if it has, you can re-formulate your topic)
(2) provides examples of search strategies used for your topic or for parts of your topic.
NOTE: You should also search for other types of studies on your topic to make sure there is enough literature to conduct a systematic review. For example, for intervention or therapy studies, for which randomized controlled trials are the gold standard in terms of evidence, you want to find at least a few RCT's on your topic to make sure a full systematic review is feasible.
II. It is strongly recommended that you register your review with PROSPERO to help make sure the same search question wasn't / isn't being investigated by others and that no one else will start a systematic review on this topic while you're conducting one.
III. As obvious as it sounds, a systematic review / meta-analysis must be conducted in a systematic manner, and a well-defined and well-constructed protocol will get you started on the right foot! Make sure to consult at least one set of guidelines (e.g. PRISMA, Cochrane) to help develop your protocol:
- PRISMA
PRISMA stands for Preferred Reporting Items for Systematic Reviews and Meta-Analyses. It is an evidence-based minimum set of items for reporting in systematic reviews and meta-analyses. The PRISMA Statement consists of a 27-item checklist and a four-phase flow diagram (available as PDF files in the right-hand column).
- Cochrane Handbook for Systematic Reviews of Interventions
The official document that describes in detail the process of preparing and maintaining Cochrane systematic reviews on the effects of healthcare interventions.
- Institute of Medicine: Finding What Works in Health Care
The IOM recommends 21 standards for developing high-quality systematic reviews of comparative effectiveness research. The standards address the entire systematic review process from the initial steps of formulating the topic and building the review team to producing a detailed final report that synthesizes what the evidence shows and where knowledge gaps remain.
Formulating the question
Construct a narrow foreground question using the PICO format (worksheet available in right-hand column). Or, use the following formula as a template:
In Patients [include any significant demographics] with [specify Problem],
does [specify Intervention] or [specify Comparison, if any]
affect [specify Outcome]?
For example: In patients with type 2 diabetes, does a low-carbohydrate diet or a low-fat diet improve metabolic control and quality of life?
The type of studies you should include in your literature search will depend on the type of question being asked:
| Question Type | Suggested Study Types |
| Diagnosis | RCTs > prospective studies |
| Therapy | RCTs > cohort studies > case-control studies |
| Etiology | RCTs > cohort studies > case-control studies |
| Prognosis | cohort studies > case-control studies |
Initial search considerations
- Decide which databases to search: general biomedical databases (PubMed/MEDLINE, Scopus/EMBASE, CINAHL), subject-specific databases (PsycINFO, ERIC), and/or grey literature (ClinicalTrials.gov, Conference Proceedings Index, Dissertations).
- Develop a list of keywords and subject headings for each concept in your question. Search engines are only as smart as you tell them to be! You will have to think of all the synonyms that could be used to describe each concept in your question, because different authors may use different terms for the same condition or population (e.g. heart attack / myocardial infarction / cardiovascular stroke). HINT: Use the Concept Table to help construct your list of terms.
- Computers operate using mathematical language, so you will have to combine your search terms in a certain order (using AND, OR, NOT), use quotes around phrases, parentheses around separate concepts, truncation symbols, etc.
Managing your results
- For each database searched, save the exact search strategy used, date of search, and number of results.
- Save the list of results in Excel format (XLS, TXT) and/or the format required for your citation management progam (EndNote, Zotero).
For more information about these programs, check out our Citation Management guide.
-
RayyanA free online tool that can be used for independent screening and coding of studies in a systematic review. Rayyan uses tagging and filtering to code and organize references.
-
abstrackrA free and open source program from Brown University. Allows participants to collaboratively screen and annotate abstracts for relevance; will import abstracts directly from PubMed (using PMIDs) or from a reference manager.
-
colandrFree open access, machine-learning assisted tool for screening and data extraction.
-
ExcelExcel (Baystate license) can be used in the screening and data extraction stages of the systematic review process. Customized workbooks and spreadsheets can be designed and lists of references can be exported from Endnote and other citation managers for screening.
Subject Guide
Step-by-Step Guides
-
Piecing Together Systematic ReviewsFive-part series of video tutorials from the Network of the National Library of Medicine.
-
Steps in a Systematic ReviewFrom Duke University's Medical Center Library.
-
Guide to Evidence Synthesis: Steps in a Systematic ReviewFrom Cornell University Library
Search Forms
Use these forms to better define your own research question and search strategy.
-
Record Keeping TipsHelpful tips on how to keep track of your searches and manage your citations.
-
PICO WorksheetFormulate your question in PICO format and come up with initial search terms for each concept.
-
Concept TableRecord which databases to search and list keywords/subject headings for each concept in your research question.
Search Strategy Helpers
-
Yale MeSH AnalyzerEnter PubMed ID numbers of relevant articles to view a list of MeSH headings and keywords that you may want to use in your final search strategy.
-
Polyglot: Search Strategy TranslatorTranslate your complete search strategy from one database to another with the proper formatting/syntax (e.g. from PubMed to PsycINFO).
-
ISSG Search FiltersSearch filters from the InterTASC Information Specialists' Sub-Group.
-
Ovid Search FiltersFilters to help you limit your Ovid search to a specific category such as diagnosis or children.
-
McGill LibrarySearch filters and other helpful tools from McGill University library.
-
Harvard Countway LibraryVerified search filters to limit your search by study design (e.g. RCT's).
Systematic Review / PRISMA Resources
-
PRISMA 2020 ChecklistA helpful checklist to use when formulating your study protocol,
-
PRISMA 2020 Flow DiagramA flow diagram for recording your references.
-
CIting PRISMAHow to cite PRISMA in your paper.
-
PRISMA Flow Diagram GeneratorPlug in your search and screening results and this software will create the diagram for you!
-
Equator NetworkReporting guidelines by study type and other resources to help you write and publish your health-related research.
-
SR ToolboxA searchable database of tools to help with protocol development, screening, quality assessment, reporting standards, and guidelines.
-
Translating Search Terms for Google ScholarIf using Google Scholar in your systematic review, check out this video tutorial on translating the search terms correctly and using Publish or Perish for easier export to EndNote, etc. (Tutorial from University of Alabama at Birmingham Libraries)
Flow diagram of systematic review process
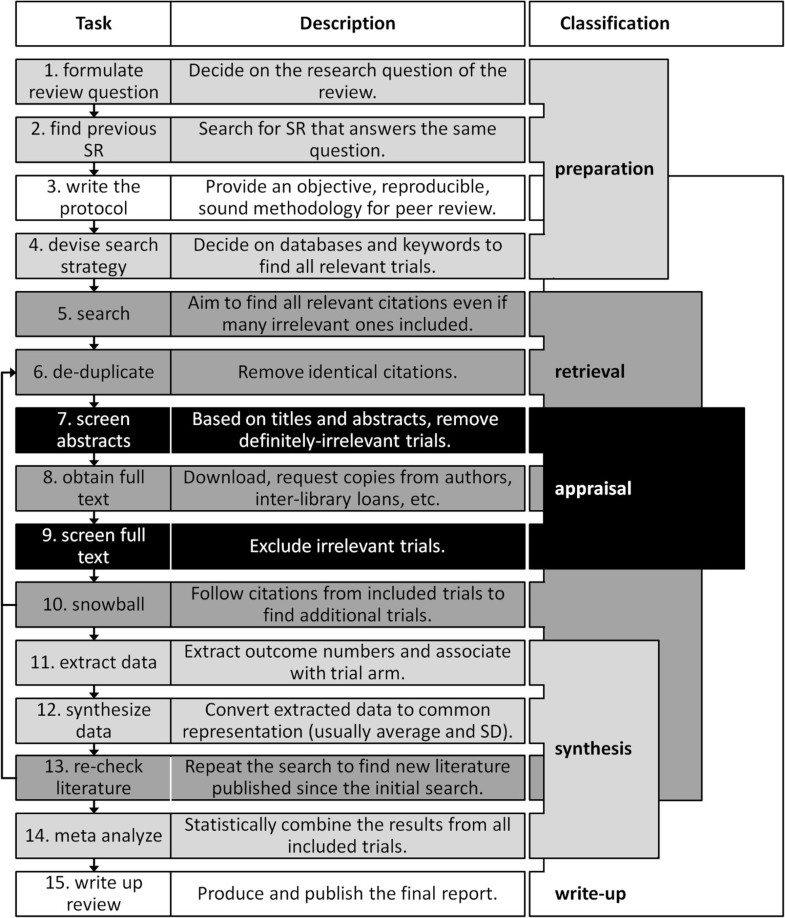
NOTE: Step 14 is for researchers performing meta-analyses only.
Tsafnet G, Glasziou P, Choong MK, et al. Systematic review automation technologies. Systematic Reviews 2014;3:74. http://www.systematicreviewsjournal.com/content/3/1/74

